Meeting the Need for High-Volume Drug Delivery with On-Body Bolus Injectors
Tsachi Shaked | 27.09.2020

In this article, Tsachi Shaked, Managing Director and Chief Business Officer at E3D, argues that on-body injectors present a safe and viable solution for high-volume drug delivery.
As the covid-19 crisis continues, many regular hospital visits and scheduled surgeries have been cancelled or put on hold, due to concerns about over-burdened hospital resources as well as fear of infection. Many mandatory hospital visits can be eliminated with the use of an on-body bolus injector which can handle drug volumes of 3 mL and over.
An established player in the injectable medications market, Elcam Drug Delivery Devices (E3D) is working to create on-body delivery devices for subcutaneous or intramuscular delivery of medications outside a clinical environment. Creating a convenient bolus injector for home use spares patients a tiring hospital trip. Instead, they can rest comfortably at home while the on-body injector does its job (Figure 1). As healthcare resources are stretched increasingly thinly, it can provide welcome relief by providing a cost-effective alternative to outpatient treatment.
“An on-body injector that can deliver high-volumes and viscous drugs slowly over a predetermined period ensures easy handling, safety, reduced pain and leak-free delivery and represents a viable alternative to autoinjectors.”
WHY USE AN ON-BODY BOLUS INJECTOR?
 For self-administration, the rule of thumb for the length of time a patient can hold an autoinjector is 15 seconds. Because injections of 3 mL and more can take longer than 15 seconds, using a self-administered injection means longer holding time. With much higher volumes, subcutaneous tissue is limited in its ability to handle the fast injection of large volumes – and injecting large volumes can result in the drug leaking as well as causing the patient unnecessary pain. An on-body injector that can deliver high-volumes and viscous drugs slowly over a predetermined period ensures easy handling, safety, reduced pain and leak-free delivery and represents a viable alternative to autoinjectors. E3D’s on-body bolus injector (OBI) (Figure 2) delivers bolus subcutaneous injections at the desired injection time, as cost-effectively as possible and with minimal need for patient intervention. It provides automated drug delivery in the familiarity, comfort and convenience of the patient’s home, eliminating the risk of exposure to carriers of covid-19 or other infectious diseases. It’s also likely to be well accepted by patients. Recent research has shown that patients are showing increasingly high satisfaction with self-administered antibiotics using antibiotic elastomeric pumps.1
For self-administration, the rule of thumb for the length of time a patient can hold an autoinjector is 15 seconds. Because injections of 3 mL and more can take longer than 15 seconds, using a self-administered injection means longer holding time. With much higher volumes, subcutaneous tissue is limited in its ability to handle the fast injection of large volumes – and injecting large volumes can result in the drug leaking as well as causing the patient unnecessary pain. An on-body injector that can deliver high-volumes and viscous drugs slowly over a predetermined period ensures easy handling, safety, reduced pain and leak-free delivery and represents a viable alternative to autoinjectors. E3D’s on-body bolus injector (OBI) (Figure 2) delivers bolus subcutaneous injections at the desired injection time, as cost-effectively as possible and with minimal need for patient intervention. It provides automated drug delivery in the familiarity, comfort and convenience of the patient’s home, eliminating the risk of exposure to carriers of covid-19 or other infectious diseases. It’s also likely to be well accepted by patients. Recent research has shown that patients are showing increasingly high satisfaction with self-administered antibiotics using antibiotic elastomeric pumps.1
Operating the OBI takes only four simple steps:
1. Peel off liner
2. Attach to skin at injection site
3. Press button to start injection
4. Peel off after injection completed.
Its key features are:
- Sterile
- Prefilled, preloaded and ready to use
- Adjustable injection time
- Variable injection volumes – from 3 mL to 40 mL
- Audible and visual indications for injection start and end as well as injection progress
- Full control of injection states
- Injection data transmission.
- Needle safety – needle hidden
Because the smart injector device can be configured for wireless communication, full monitoring of comprehensive injection data can be made available – the physician is able to monitor the patient status through a mobile app, ensuring that the dosage was administered in full at the correct time.
“Because the smart injector device can be configured for wireless communication, full monitoring of comprehensive injection data can be made available.”
MAKING EARLY ADOPTION EASY
While there is often a small learning curve with autoinjectors, E3D addressed the usability issue by replicating a user experience already introduced to market. The preloaded ready-to-use OBI devices are easy to use. Key features considered during the design process included:
- User focused and ergonomic – the device can be easily applied to the patient’s skin because of its size.
- Automated delivery – delivery of bolus injection (needle penetration) is automatically performed after being applied to the patient’s skin and pressing the injection button.
- Stable attachment – device design and size ensure stable and safe attachment to the patient’s body that cannot be easily dislodged.
- Simplified design – the device size has been reduced and the design simplified to enable cost-effective manufacture.
VERSATILE TECHNOLOGY PLATFORM
The OBI device technology platform (Figure 3) offers significant advantages in the delivery of other injectables, as it is cost effective, compact, light, inherently robust, economical and readily scalable for any dose volume. The OBI platform can easily be scaled to deliver a liquid bolus of up to 40 mL. During early drug lifecycle development, dosage volume is frequently high. As development progresses, pharma companies are able to increase concentration and reduce dose volume. Since the device is well suited to delivering high-volume viscous liquids, it addresses
an increasingly prevalent requirement for a versatile platform to accommodate the needs of emerging drug products.
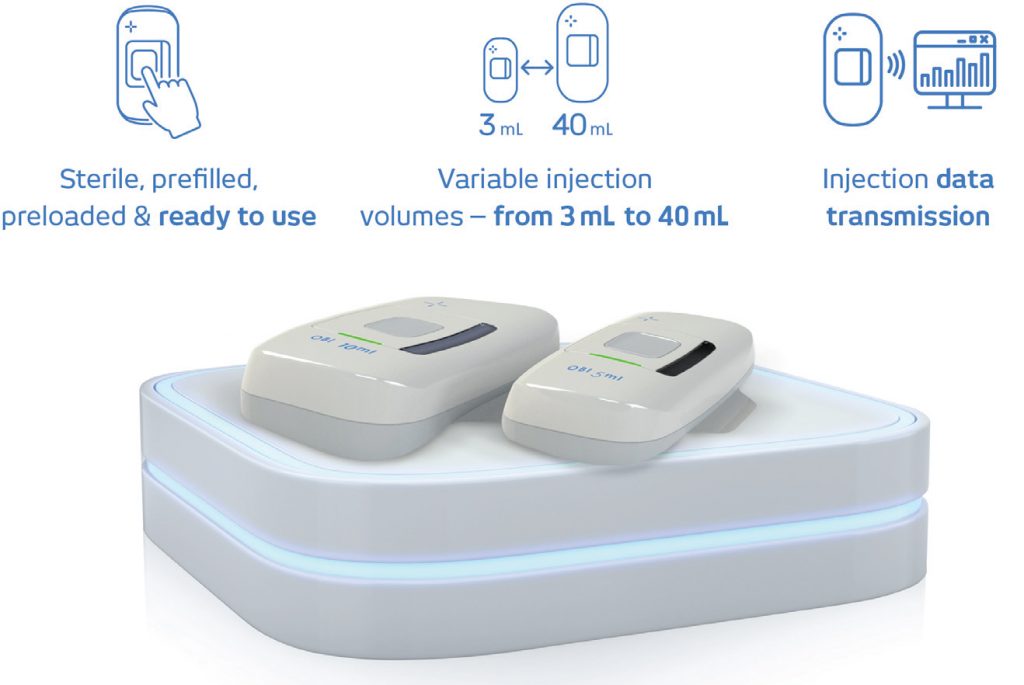
Figure 3: The OBI platform
THE FUTURE OF ON-BODY TECHNOLOGY
On-body injectors are set to represent an increasingly attractive alternative to handheld injector devices for higher dose volumes and viscous drugs. The compact, robust, flexible and cost-effective OBI platform can provide the required capabilities to meet the needs of specific drugs, therapies and patient populations, creating significant advantages for a broad range of drugs for
a wide variety of indications including autoimmune conditions, oncology and multiple sclerosis. Its versatility is set to provide significant added value to pharma companies seeking cost-effective on-body alternatives for large-volume and/or high-viscous drug applications. With more injectables requiring high dose volumes and with more of these drug products having elevated
viscosities, on-body injector devices will have an increasingly important role to play in providing effective, safe and convenient patient care.
Most importantly, the OBI is another step in E3D’s quest to free patients from having to visit hospitals, especially as the impact of the coronavirus crisis continues to be felt the world over(Figure 4).

“The OBI is another step in E3D’s quest to free patients from having to visit hospitals.”
ABOUT THE COMPANY
The Elcam Drug Delivery Devices (E3D) portfolio encompasses a wide range of injectables produced in the company’s manufacturing facilities in Europe, the US and Israel. These devices include single- and multi-use, spring-powered autoinjectors designed for 1 mL and 2.25 mL prefilled syringes; wearable injectors for bolus, high-volume and viscous drug delivery; electromechanical and mechanical “smart” injectors with wireless connectivity; autoinjectors for viscous formulations; emergency-use injector devices; and injectors with both automated and manual reconstitution for lyophilised products.
REFERENCES
Saillen L et al, “Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps”.
Eur J Clin Microbiol Infect Dis,
2017, Vol 36(8), pp 1387–1392.