Flexi-Q 2-step Family
Tsachi Shaked | 28.10.2021

There is a growing need for devices which facilitate the injection of parenteral drugs outside of a healthcare environment. This is in part driven by the numbers new biologic drug products, and corresponding biosimilars, for a wide range of long-term therapies to address chronic conditions and continued launches of biosimilars, typically requiring subcutaneous injection, which can be self-administered. In addition, there is an increase in the number of drug products which can be used for first line treatment in case of life-threatening emergencies in both military and civilian settings – and which generally require intramuscular injection administration either by the casualty themselves or by a non-clinician who is with the casualty. These demands have fuelled the development and introduction of a number of auto-injector technologies which are intended to enable the safe, comfortable and effective injection of such drugs.
Whilst it was generally hitherto considered that a 1mL dose volume was the maximum that could be delivered with such devices in a duration of 10 seconds or less, this view has been largely discounted with the introduction in recent years of devices which are capable of injecting up to 2.25mL from a standard pre-filled syringe (PFS).
Elcam Drug Delivery Devices (E3D) is an established developer and supplier of auto-injector devices whose business model has previously involved the creation of a bespoke solution, based on its patented button-activated, 3-step Flexi-Q PFS platform. Whilst this approach can ensure a pharmaceutical company partner of a unique device design in case of each product, it does essentially require that each product a full development project – with corresponding implications for cost, timelines to commercialisation and attendant project risk. It also means that a dedicated manufacturing facility must be created for each device, with the associated capital investment and cost-of-goods implications.
In order to address these challenges of cost, timelines and risk, E3D has now developed its Flexi-Q 2-step family of standardised, off-the-shelf auto-injectors, enabling the company to offer an easy-to-use and intuitive solution for delivery of drugs in 1mL or 2.25mL PFS, subcutaneously or intramuscularly, for chronic conditions or use in emergencies – and to do so from manufacturing facilities which have already been established by E3D. In addition, specific device embodiments, which require only minimal adaptations to a small number of device components – thereby enabling product differentiation without the need for a full-scale development project
Flexi-Q 2-step Family of Auto-injectors
E3D is subsidiary of Elcam Medical ACAL, a company with 40 years’ experience in development, manufacturing and worldwide supply of medical devices.
Elcam’s manufacturing processes combine sophisticated equipment with superior facilities to ensure production of consistently high-quality products at any volume level. The company has experience in use of manual and semi-automated machines for low-volume production, as well as high-volume manufacturing and assembly incorporating fully-automated production lines and quality controls.
State-of-the-art injection mold tooling, including multi-cavity and/or two-component molds in versatile injection machines produce components in a variety of polymeric and elastomeric materials. The entire process of tooling design and manufacturing, to production, packing and shipping, is conducted under Elcam’s quality management system. Barcoded lots are stored and dispatched using computerized logistics to provide full traceability for each product shipped to customers.
E3D is focused on design, development, manufacturing and supply of injectable drug delivery devices for self-administration and use in non-clinical environments. Over the past 15 years, E3D has built a broad technology portfolio encompassing devices suitable for drugs in a range of primary containers – including auto-injectors, on-body patch pump injectors and smart devices with wireless connectivity. With device manufacturing facilities in a number of countries, E3D works closely with customers from initial design phase to regulatory approval and commercial supply to provide innovative and differentiated solutions for delivery of injectable drugs. From design to supply, the focus is on provision of devices which meet specific needs of the intended user populations.
E3D has a record of developing injectable delivery devices with and for pharmaceutical company partners, with its Flexi-Q PFS (button-activated, single use 3-step auto-injector) approved and launched in the international market as the device element a combination product with a partner’s blockbuster biologic product. E3D is also currently involved in a number of development projects with a variety of other partners and based on its various injection device technology platforms.
In order to address the growing demand for easy-to-use auto-injector which can address a wide range of therapeutic applications and which offer fast, economic and low-risk routes to market for pharmaceutical companies, E3D has now developed its Flexi-Q 2-step family, which consists of two new device platforms, both single-use auto-injectors incorporating either 1mL or 2.25mL PFS with staked needles – and which require only two user handling steps, being activated simply by pushing the auto-injector device against the skin at the appropriate injection site and not requiring any buttons or triggers to initiate the injection.
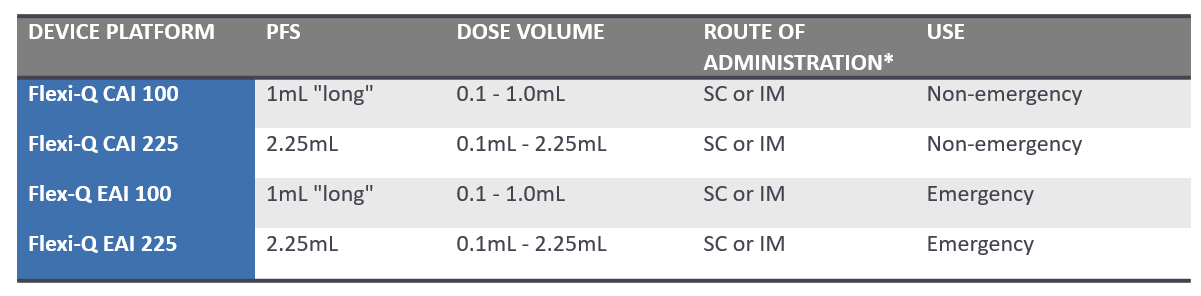
Flexi-Q CAI: a two-step, single-use auto-injector platform developed primarily for use in chronic therapies.
Flexi-Q EAI: a two-step, single-use auto-injector platform, developed primarily for use in emergency and rescue applications – and designed to meet corresponding reliability requirements according to published regulatory guidance for emergency-use injector devices.

User handling steps for both Flexi-Q CAI and Flexi-Q EAI devices are:
- Step 1: pull and remove the Safety Cap from the device
- Step 2: push the end of the device against the intended site of injection
Once pushed against the tissue to activate the device, needle insertion and injection of drug are both performed automatically, with the user receiving feedback on injection status and progress by means of audible, visual and tactile cues. With the needle hidden from view at all times and with a Needle Guard that automatically and securely locks over the used needle after use, these devices address a patient’s potential anxiety over their injection and also eliminates the risk of needlestick injury following use of the auto-injector.
Flexi-Q CAI and Flexi-Q EAI can both be configured for either subcutaneous or intramuscular injection and can each incorporate either 1mL “long” or 2.25mL pre-filled syringes (PFS).
Prior to development of Flexi-Q CAI and Flexi-Q EAI platforms, E3D’s approach has been to create bespoke device embodiments for a given pharmaceutical company partner, with this requiring considerable development resources and the establishment of dedicated manufacturing facilities in each case. Such an approach not only results in additional costs (both in development and capital expenditure) and time, but also carries additional risks associated with the undertaking of a full development projects.
The development strategy for Flexi-CAI and Flexi-Q EAI has been for E3D to invest in and establish appropriate manufacturing capabilities for a standardised, off-the-shelf version of each device in – parallel with the development activities for the device platforms. This investment by E3D enables pharmaceutical company partners to benefit from access to a full solution of patented device technology, manufacturing and supply – thus providing access to E3D’s proprietary technology, shortening time to market, reducing development costs and eliminating much of the project risk. In addition, use of a common manufacturing facility across a number of different devices creates opportunities for greater manufacturing efficiencies and cost benefits arising from equipment utilisation and economies of scale – resulting in unit cost advantages.
This is not to say that partners cannot have elements of devices which are bespoke and which facilitate differentiation for their products. Parameters such as dose volume, delivery duration, drug viscosity, external device geometry, colours and branding can be accommodated. Such adaptation to the standardised device will require some element of device design and development, as well as some degree of dedicated component manufacturing – but the standardised device is configured to accommodate such without altering the fundamental design and without requiring a full development project (with the costs and timelines that this would otherwise require).
Production of Flexi-Q 2-step auto-injector sub-assemblies is carried out at Elcam facilities and two sub-assemblies (front and rear) for each device are shipped to a partner’s preferred facility (typically in-house or at a contractor) for final assembly with its drug-filled syringe. E3D facilitates the selection and installation of suitable final product assembly systems from established vendors, according to commercial-scale manufacturing capacity requirements.
The development of the Flexi-Q 2-step auto-injector family represents an exciting new phase of E3D’s evolution, going beyond its business model solely based on design and supply of bespoke devices and enabling it to offer cost-effective solutions which address a wide range of clinical applications – and with established manufacturing capabilities providing a fast route to commercialisation for its partners and their injectable drug products.